Organophosphates also known as the OPs. The word
organophosphate is derived from the combination of two words; ‘organic
compounds’ and ‘phosphate group’. There is no known clinical use of these
chemicals. However, the main uses are as agricultural insecticides and fungicides;
used in household and garden sprays for flies and other insects.
 |
| Organophosphates (Cover) |
Usage
Organophosphate
insecticides are commonly used for small animals as flea and tick powders,
sprays, foggers, shampoos and dips, and formerly, as systemic insecticides. They
are also frequently used as household, garden, and farm insecticides. OPs, in
general, have short term persistence and limited residual activity. They are
more toxic to vertebrates than other insects. OPs have replaced the banned
organochlorines (OC) and are a major cause of animal poisoning.
History
OPs
were first synthesized in the 1850’s but the modern products were developed in
Germany in the 1930’s. Their insecticidal qualities were first observed in
Germany during World War II in the study of the extremely toxic OP nerve gases sarin,
soman, and tabun. These
were poisonous to mammals, birds, reptiles and fish. OPs are the
components in chemical weapons called Nerve Gases.
Organophosphates
have less residual effect than Organochlorines and their break down in the
environment is faster. Organophosphates damage the nervous system and are
highly toxic at small doses.
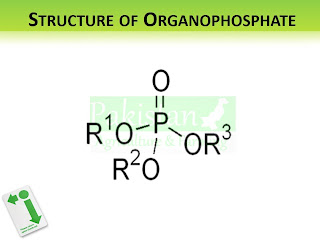 |
| Structure of Organophosphates |
MODE
OF TOXICITY
The
common mode of toxicity is ‘acute toxicity’ through skin, inhalation and
ingestion. OPs affect the acetylcholine receptors in the nerve endings.
Normal
Transmission of acetylcholine (ACh)
ACh
is released in the axon terminal and crosses the synaptic cleft. ACh binds to a
receptor in the post-synaptic membrane. Acetylcholinesterase (AChE) stops the
action of ACh.
Transmission
with Nerve Agent (NA)
ACh
is released from the axon terminal and crosses the synaptic cleft. ACh then
binds with a receptor on the post-synaptic membrane. Nerve agents (NA) blocks
the ability of AChE to stop the action of ACh; as a consequence, ACh continues
to work and more ACh builds up in the synapse.
Symptoms
of Poisoning
|
Neuromuscular Effects
|
Autonomic Nervous
System Effects |
Central Nervous
System Effects |
|
Twitching
Weakness
Paralysis
Respiratory failure
|
Reduced Vision
Sweating
Diarrhea
Nausea
Abdominal pain
Vomiting
|
Headache
Coma
Respiratory arrest
Confusion
Depression
Respiratory
|
Derivatives of OPs
1.
Aliphatic derivatives
2.
Phenyl derivatives
3.
Heterocyclic derivative
1.
ALIPHATIC DERIVATIVES
Aliphatic
are simple phosphoric acid derivatives bearing short carbon chains. Some of
these are malathion, trichlorfon, dicrotophos, oxydemetonmethyl, disulfoton, dichlorvos,
methamidophos and acephate.
2. PHENYL
DERIVATIVES
Phenyl
(or benzene) ring with one of the ring’s hydrogen displaced by attachment to
the phosphorus moiety and other hydrogen atoms frequently displaced by -Cl, -NO2,
-CH3, -CN, or S. These are generally more stable than the aliphatic
OPs so their residues last longer. Examples include methyl parathion and
profenofos (Curacron®).
3.
HETEROCYCLIC DERIVATIVES
These
are also ring structures but differ in having one or more carbon atoms
displaced by O, N, or S. These are complex molecules and generally have much
long lasting residues than many of the aliphatic or phenyl OPs. They also have
many breakdown products which makes it difficult to measure their residues in
the laboratory. Examples include diazinon,
azinphosmethyl and methidathion.
ADVANTAGES
AND DISADVANTAGES
Advantages
1.
Used as insecticides, fungicides and herbicides.
2.
Broad spectrum of activity against a number of pests.
3.
Poisons stomach and has contact action.
4.
Many also possess trans-laminar or systemic action.
5.
Biodegradable and converted to non-toxic metabolites.
6.
Less possibility of pollution.
7.
Poses low chronic toxicity.
8.
Very economical and used in smaller dosages.
Disadvantages
1.
Some pose bad order. For example, Malathion.
2.
Some pose high acute mammalian toxicity.
3.
Require special training for application, for example phorate and disulfotan.
TOXICITY
TO HUMANS
1.
Respiratory difficulty
2.
Muscle Weakness
3.
Hypertension
TOXICITY
TO ANIMALS
1. Restlessness
2. Hyperexicitability
3. Convulsion
4. Paralysis
5. Death
BIOMAGNIFICATION
Organophosphates
break down quickly in the environment and their residues on crops are less. They
are also not stored in animal tissue, so biomagnification has not been a
problem for either of these reasons, their use has greatly reduced the hazard
to non-target species.

